Trends in Cognitive Sciences Review: “Brain Oscillations and the Importance of Waveform Shape
January 5, 2017 by Bradley Voytek
[Voytek’s note: As part of the lab’s ongoing “Why we think our research is cool series,” neurosciences PhD student Scott Cole summarizes and contextualizes his most recent review paper, Brain oscillations and the importance of waveform shape, published in Trends in Cognitive Sciences. This paper grew out of a talk I gave in April 2016 at the Cognitive Neuroscience Society conference in New York as part of a special symposium titled “The role of amplitude, phase, and rhythmicity of neural oscillations in top-down control of cognition.” During that talk I discussed two recent lines of work in the lab, exemplified by two papers currently under review. The first, by Scott is Nonsinusoidal oscillations underlie pathological phase-amplitude coupling in the motor cortex in Parkinson's disease; the second, by Cognitive Science PhD student Richard Gao, is Inferring synaptic excitation/inhibition balance from field potentials. Both outline ways in which we might be able to extract more information from common brain imaging/recording techniques such as LFP, EEG, MEG, and ECOG. After that talk, I was invited to submit a review on the topic. Hence the current paper!] The Nobel Prize winning method of recording the electrocardiogram (ECG), and associated discovery of the PQRST complex in the ECG signal, resulted in a significant shift in the understanding of cardiac physiology. Once the ECG was demonstrated, researchers began to focus on identifying the physiological generators for each of the temporal subcomponents of the PQRST complex. These subcomponents, which include the slower P- and T-waves along with the sharper QRS complex, were hypothesized to reflect different aspects of cardiac electrophysiology (see Figure below). In fact, it was the careful consideration of how these complexes were generated that guided discoveries of new cardiac cell types and their interactions, in turn leading to a better understanding of the physiological origins of cardiac disease, reflected by abnormalities in the ECG signal.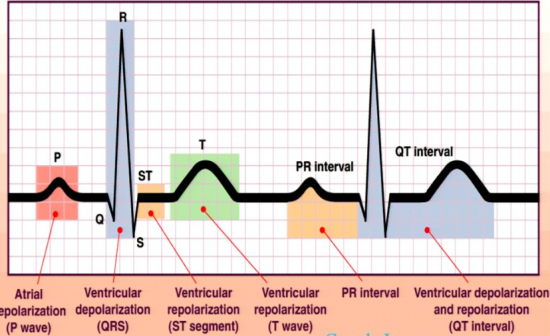
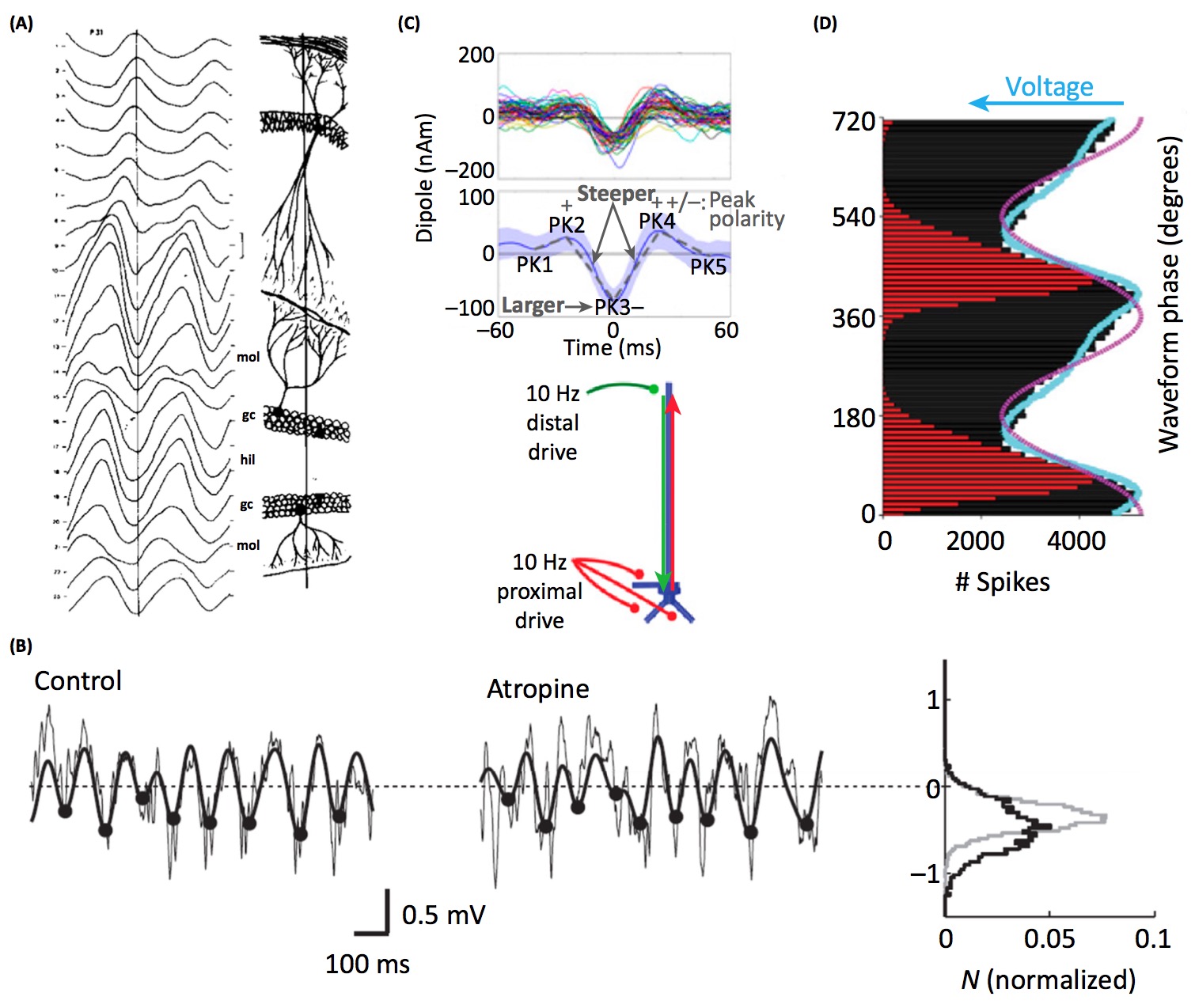
- Brain rhythms are different shapes in different brain regions. Perhaps this reflects differences in neural computation, despite sharing a common fundamental frequency (see panel A below). [Buzsaki et al. 1986]
- Manipulating the concentration of neurotransmitters modifies features of oscillatory waveforms. Perhaps the waveform shape of some oscillators reflect properties of neuromodulation (see panel B below). [Hentschke et al. 2007]
- Cortical beta oscillations have a stereotyped waveform. The waveform properties are predicted by a model of synchronous distal and proximal excitatory synaptic synchrony (see panel C below). [Sherman et al. 2016]
- The sawtooth-like waveform of hippocampal theta oscillations fits the firing rate histogram of the local neurons better than a sinusoid. Perhaps the waveform shape of some oscillators reflect the temporal properties of local spiking (see panel D below). [Belluscio et al. 2012]
- Motor cortical beta oscillations are sharper in untreated patients with Parkinson’s disease. Perhaps this reflects hypersynchronization of the beta generator. Perhaps waveform shape may be a useful biomarker for disease. [Cole et al. 2016]
- Hippocampal theta oscillations are more asymmetric while a rat is running compared to during immobility, REM sleep, or lever pressing [Belluscio et al. 2012]. Hippocampal theta oscillations are also more asymmetric during an experience which a rat subsequently remembers [Trimper et al. 2014]. Perhaps changes in oscillatory waveform reflects a change in the mode of neural computation.
- Rhythmic electrical stimulation has different effects depending on the shape of the stimulating waveform. Most notably, electroconvulsive therapy is more effective using a rectangular waveform compared to a sinusoidal waveform. [Weiner et al. 1986]